Graphite: A Trusted Performer
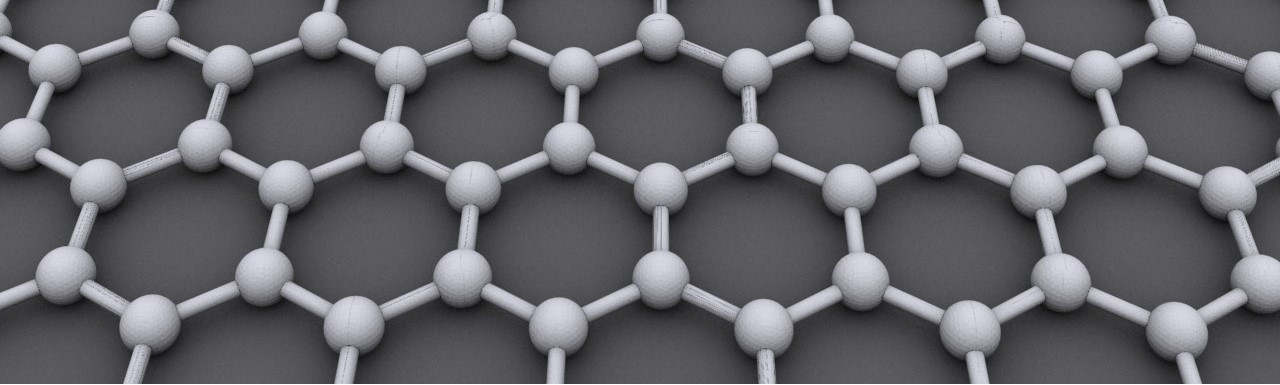
Graphite is a naturally occurring form of carbon, made up of layers of carbon atoms stacked on top of each other. These layers are loosely bonded, allowing them to slide past one another, which gives graphite its well-known slippery feel. This layered structure is responsible for its excellent thermal and electrical conductivity, as well as its stability under high temperatures.
Practical Uses and Advantages:
Graphite is widely used for managing heat in electronics and industrial equipment. With a thermal conductivity of up to 2000 W/m·K along its layers, it efficiently moves heat away from components in devices like heat sinks and thermal pads.
It is also a great electrical conductor, with a conductivity of around 2.5 × 10^4 S/m, making it a key material in batteries and fuel cells.
Because it can withstand temperatures up to 3,000°C in inert atmospheres, graphite is used in high-temperature applications such as industrial furnaces and heat-resistant coatings (Pierson, 1993).
Its role in energy storage is just as important. As the primary anode material in lithium-ion batteries, graphite offers a theoretical specific capacity of 372 mAh/g, making it a reliable and cost-effective choice for powering devices.
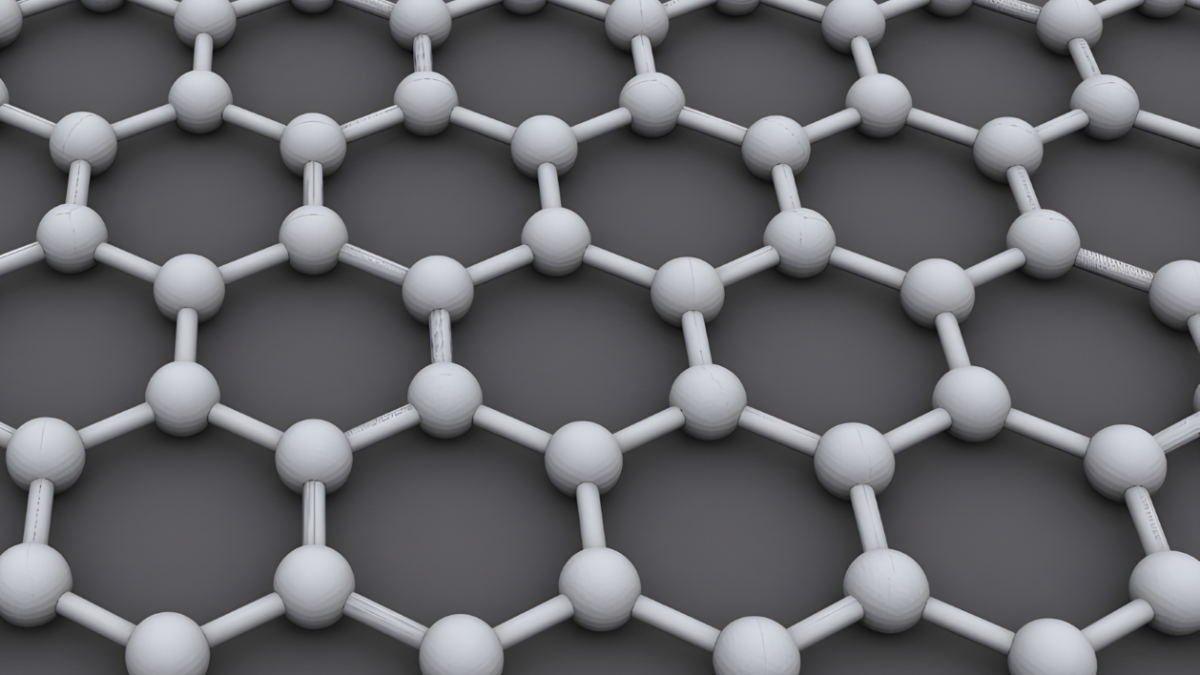
Graphene: A Material of the Future
Graphene is a single layer of carbon atoms arranged in a hexagonal pattern. This ultra-thin structure gives it extraordinary properties, including incredible strength, flexibility, and conductivity. Since its discovery in 2004, graphene has been at the centre of technological breakthroughs across multiple industries.
Key Benefits and Uses:
Graphene is one of the strongest materials known, with a tensile strength of 130 GPa—about 200 times stronger than steel by weight. Despite its toughness, it remains extremely lightweight and flexible, making it ideal for a range of advanced applications.
It is also an exceptional electrical conductor, reaching up to 10^8 S/m, which makes it a perfect candidate for high-speed transistors, flexible electronics, and ultra-thin conductive coatings.
Graphene’s thermal conductivity is equally impressive at ~5,000 W/m·K, making it one of the best heat-conducting materials available. This property is particularly valuable for advanced cooling solutions in electronics.
Another standout feature is its transparency. Graphene absorbs only 2.3% of visible light, making it an excellent choice for touchscreens, flexible displays, and even solar cells.
Energy storage is another area where graphene shines. Graphene-based supercapacitors have demonstrated energy densities of 85.6 Wh/kg, offering the potential for faster-charging, longer-lasting power sources.
In medicine, graphene’s biocompatibility and electrical properties are being explored for applications like biosensors, drug delivery, and regenerative medicine.
Graphite vs. Graphene: A Side-by-Side Comparison
Although graphite and graphene share the same basic carbon structure, their properties differ significantly. Graphite is a bulk material made up of multiple layers, while graphene is just a single layer. This difference gives graphene superior strength, conductivity, and flexibility, whereas graphite remains a practical and affordable material for large-scale applications.